SIC 1474
POTASH, SODA, AND BORATE MINERALS
This category covers establishments primarily engaged in mining, milling, or otherwise preparing natural potassium, sodium, or boron compounds. Establishments primarily engaged in mining common salt are classified in SIC 1479: Chemical and Fertilizer Mineral Mining, Not Elsewhere Classified.
NAICS Code(s)
212391 (Potash, Soda, and Borate Mineral Mining)
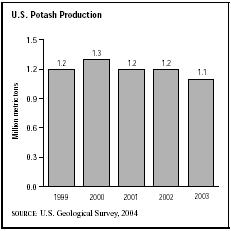
In 2003 the majority of potash production took place in New Mexico, where three mines were in operation. Michigan and Utah also had potash production facilities. In the 2003 crop year, about 1.1 million metric tons of potash were produced in the United States, down from 1.2 million metric tons the previous year. The fertilizer industry accounted for about 85 percent of U.S. potash sales, and the chemical industry used about 15 percent. Roughly 60 percent of the potash extracted in 2003 was produced as potassium chloride, with potassium sulfate and potassium magnesium sulfate—both for fertilizing certain crops and soils—representing the remainder of potash production. Of the world's potash reserves, the largest percentage was located in Canada and Russia. Canada accounted for 93 percent of U.S. potash imports, which totaled 4.5 million metric tons in 2003. The United States relied on imports for roughly 80 percent of its total consumption.
As a fertilizer, potash was used for such crops as soybeans, tobacco, potatoes, sugar beets, and corn, and the potash mining industry was thus subject to the sea-sonal
fluctuations of the agricultural market. Before the Civil War, U.S. production of potash involved removing it from wood ashes through a leaching process. In 1916 potash began to be extracted through crystallization from saltwater lake brines in southern California, and later potash was mined from deposits discovered in New Mexico, which became the primary source of domestic potash in the United States. Following the oil crisis of 1979, demand for potash in developed nations declined, but in 1986 began a steady climb of 140,000 short tons a year.
By the 1990s the world's potash markets were in oversupply, and in the late 1990s and early 2000s, potash producers were forced to operate at reduced capacity. In addition, potash production was affected by economic crises afflicting Asia, as demand from Asia for imported grains declined. In fact Potash consumption in Asia was expected to decline 9 percent in 2003. Based on data from the U.S. Geological Survey, U.S. consumption of potash declined from 5.6 million metric tons in 2001 to 5.3 million metric tons in 2003. Exports over that time period rose from 366,000 metric tons to 370,000 metric tons, with the majority going to Latin America.
By 2003 there were only four potash-producing companies in operation in the United States, down from 10 companies in 1996. These four companies worked a total of seven production facilities. One of these firms, Mississippi Chemical Corp., filed for Chapter 11 bankruptcy protection in May of 2003 due to rising raw materials costs, which made it difficult to turn a profiton nitrogen and phosphorus. In March of 2004, Mississippi Chemical agreed to sell its two remaining potash mines in New Mexico. Another potash producer, IMC Global Inc., had acquired Western Ag-Mineral in 1997 and Harris Chemical Group, Inc. in 1998. IMC posted sales of $2.19 billion in 2003; by then, the firm operated six potash mines. In January of 2004 Cargill Inc. announced its intent to purchase IMC. Other consolidations in the late 1990s had led to Canadian-based Potash Corporation's ownership of the Moab Salt Company in Utah and Reilly Industries, Inc.'s ownership of the Reilly-Wendover potash site.
The soda ash mining segment of the industry, the world's largest, consisted of six U.S. companies in Wyoming and California, which in 2003 produced 10.6 million metric tons of soda ash. Soda ash, a form of sodium carbonate, is used in the production of such chemicals as sodium bicarbonate, sodium sulfate, sodium chromate, sodium phosphate, sodium silicate, potassium chloride, potassium sulfate, sodium sulfite, sodium trip-olyphosphate, and chemical caustic soda. In 2003 the most common end uses of these soda ash products were glass (49 percent); chemicals (26 percent); soap and detergents (11 percent); distributors (5 percent); flue gas desulfurization (2 percent), pulp and paper and miscellaneous (2 percent each); and water treatment (1 percent). Other applications of this "non-table salt" group of sodium or saline minerals were the production of photographic darkroom materials and wood fibers for the manufacture of wrapping paper and carton board, and the processing of textile fibers, dye manufacture, and leather tanning. Alum, Glauber's salt, and trona were other forms of sodium salts mined by industry firms. Sodium sulfate in particular was used as a substitute for salt in the dyeing processes of the textiles industry and in the glass, powdered laundry detergent, and pulp and paper industries.
After struggling with the impact of economic turmoil in Asia in the late 1990s, global demand for soda ash began to climb in the early part of the twenty-first century; as a result, U.S. production grew steadily, rising from 10.2 million metric tons in 2000 to 10.6 million metric tons in 2003. U.S. soda ash exports also increased from 3.9 million metric tons to 4.4 million metric tons over the same time period. In the early 2000s, China revealed plans to boost capacity at its soda ash facility in Weifang by 600,000 tons and to built a synthetic soda ash plant in Zhejiang with an annual capacity of 900,000 tons. Analysts predict that China will supercede the United States as the largest soda ash producer in the world by 2004.
Foreign investment in the U.S. soda ash industry increased throughout the 1990s. In 1981 foreign investment accounted for only 10 percent of soda ash production, but by 1998 foreign investment was 46 percent of capacity. European companies owned about 22 percent of soda ash production operations in Wyoming in 1998. The percentage had been higher—35 percent—in 1995, before Rhone Poulenc SA of France sold its Wyoming plant to Korea's Oriental Chemical Industries. In late 2003 Belgium-based Solvay S.A., the leading soda ash producer in the world, bought American Soda, a soda ash joint venture formed between Williams Companies Inc. and American Alkali in 2000. The deal gave Solvay soda ash capacity in excess of 9 million tons, roughly 20 percent of worldwide soda ash capacity.
The term borate minerals encompasses such boron-producing minerals as borax and kernite and includes colemanite, ulexite, and probertite. Boron was used in gasoline, jet and rocket fuel, and as an ingredient in industrial plasticizing and dehydrating processes. Borax was used in the production of enamel for bathtubs, stoves, refrigerators, and metal signs, and as an agent for ensuring brilliance and clarity in the manufacture of glass. Other uses for borax included disinfectants, preservatives, starches, lumber treatment, detergents, fertilizers, weed killers, and metallurgical applications. Borax and other borate compounds were also used as intermediate chemicals in a wide variety of industrial processes. Colemanite and ulexite were used in glass manufacturing and in the production of glass wool used for insulation.
U.S. production of boron minerals was centered in California and handled by four companies. The three regions responsible for much of the production were Kern County, San Bernardino, and Inyo County. The four borate mineral producers were American Borate Co., Fort Cady Minerals Corp., North American Chemical Co., which was owned by IMC Global, and U.S. Borax, Inc. The United States was the largest producer of boron compounds globally in 2003 and exported roughly 50 percent of its output. The largest producer of boron ore globally was Turkey. The United States continued to import borates, primarily from Turkey and Chile. The principal end uses of boron compounds in 2003 were glass and ceramic products (78 percent), soaps and detergents (6 percent), agriculture (4 percent), fire retardants (4 percent), and other uses (9 percent).
Further Reading
"Boron." Mineral Commodity Summaries. Washington, DC: United States Geological Survey, January 2004. Available from http://minerals.er.usgs.gov/minerals .
Kostick, Dennis S. "Soda Ash." U.S. Geological Survey Minerals Yearbook. Washington, DC: U.S. Geological Survey, 2002. Available from http://minerals.er.usgs.gov/minerals .
Lyday, Phyllis A. "Boron." U.S. Geological Survey Minerals Yearbook. Washington, DC: U.S. Geological Survey, 2002. Available from http://minerals.er.usgs.gov/minerals .
Monfort, Olivier. "Soda Ash: Finding A Way Out of the Crisis." Glass, November 2000. Available from http://minerals.er.usgs.gov/minerals .
"Potash." Mineral Commodity Summaries. Washington, DC: United States Geological Survey, January 2004. Available from http://minerals.er.usgs.gov/minerals .
Searls, James P. "Potash." U.S. Geological Survey Minerals Yearbook. Washington, DC: U.S. Geological Survey, 2002. Available from http://minerals.er.usgs.gov/minerals .
"Soda Ash." Mineral Commodity Summaries. Washington, DC: United States Geological Survey, January 2004. Available from http://minerals.er.usgs.gov/minerals .
Comment about this article, ask questions, or add new information about this topic: